H2-Db binding "LSLRNPILV" at 2.60Å resolution
Data provenance
Information sections
- Publication
- Peptide details
- Peptide neighbours
- Binding cleft pockets
- Chain sequences
- Downloadable data
- Data license
- Footnotes
Complex type
H2-Db
LSLRNPILV
Species
Locus / Allele group
Epitope-specific TCRbeta repertoire diversity imparts no functional advantage on the CD8+ T cell response to cognate viral peptides.
TCR repertoire diversity has been convincingly shown to facilitate responsiveness of CD8+ T cell populations to mutant virus peptides, thereby safeguarding against viral escape. However, the impact of repertoire diversity on the functionality of the CD8+ T cell response to cognate peptide-MHC class I complex (pMHC) recognition remains unclear. Here, we have compared TCRbeta chain repertoires of three influenza A epitope-specific CD8+ T cell responses in C57BL/6 (B6) mice: D(b)NP(366-374), D(b)PA(224-233), and a recently described epitope derived from the +1 reading frame of the influenza viral polymerase B subunit (residues 62-70) (D(b)PB1-F2(62)). Corresponding to the relative antigenicity of the respective pMHCs, and irrespective of the location of prominent residues, the D(b)PA(224)- and D(b)PB1-F2(62)-specific repertoires were similarly diverse, whereas the D(b)NP(366) population was substantially narrower. Importantly, parallel analysis of response magnitude, cytotoxicity, TCR avidity, and cytokine production for the three epitope-specific responses revealed no obvious functional advantage conferred by increased T cell repertoire diversity. Thus, whereas a diverse repertoire may be important for recognition of epitope variants, its effect on the response to cognate pMHC recognition appears minimal.
Structure deposition and release
Data provenance
Publication data retrieved from PDBe REST API8 and PMCe REST API9
Other structures from this publication



Data provenance
MHC:peptide complexes are visualised using PyMol. The peptide is superimposed on a consistent cutaway slice of the MHC binding cleft (displayed as a grey mesh) which best indicates the binding pockets for the P1/P5/PC positions (side view - pockets A, E, F) and for the P2/P3/PC-2 positions (top view - pockets B, C, D). In some cases peptides will use a different pocket for a specific peptide position (atypical anchoring). On some structures the peptide may appear to sterically clash with a pocket. This is an artefact of picking a standardised slice of the cleft and overlaying the peptide.
Peptide neighbours
|
P1
LEU
GLU163
TRP167
ARG62
TYR171
TYR159
TYR59
TYR7
PHE33
MET5
GLU63
LYS66
|
P2
SER
SER24
GLU63
LYS66
TYR45
TYR159
TYR7
|
P3
LEU
HIS155
TYR159
GLU9
LEU114
TYR156
LYS66
GLN97
GLN70
SER99
|
P4
ARG
ARG62
TYR156
LYS66
GLN70
HIS155
|
P5
ASN
TRP73
TYR156
GLN97
GLN70
PHE74
PHE116
|
P6
PRO
HIS155
TRP73
ALA152
TYR156
|
P7
ILE
TRP147
SER150
LYS146
TRP73
ALA152
TYR156
|
P8
LEU
ASN80
LYS146
GLN72
TRP73
VAL76
SER77
THR143
TRP147
|
P9
VAL
THR143
TYR123
LYS146
ASN80
TYR84
LEU81
ILE142
SER77
TRP73
TRP147
|
Colour key
Data provenance
Neighbours are calculated by finding residues with atoms within 5Å of each other using BioPython Neighboursearch module. The list of neighbours is then sorted and filtered to inlcude only neighbours where between the peptide and the MHC Class I alpha chain.
Colours selected to match the YRB scheme. [https://www.frontiersin.org/articles/10.3389/fmolb.2015.00056/full]
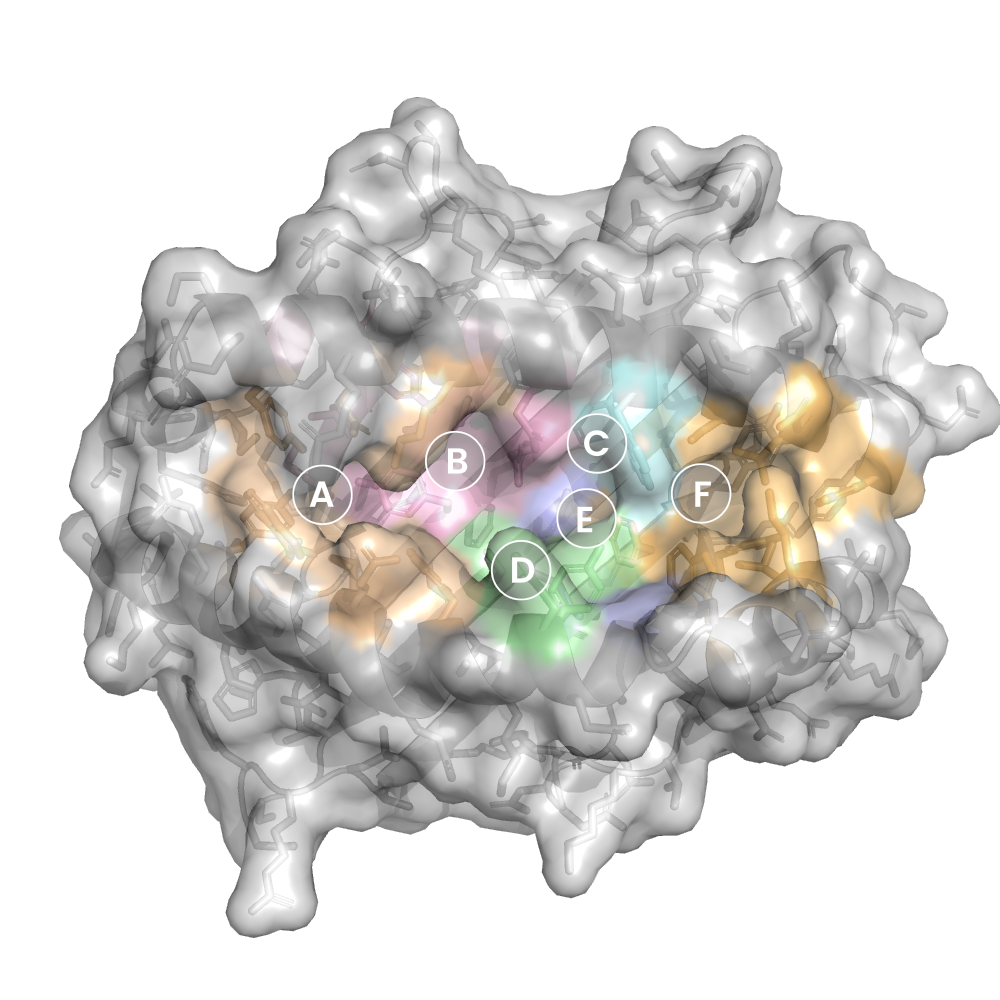

|
A Pocket
LEU159
CYS163
LEU167
LEU171
ARG5
TRP59
THR63
ALA66
PHE7
|
B Pocket
VAL24
ARG34
GLU45
THR63
ALA66
LYS67
PHE7
GLU70
THR9
GLY99
|
C Pocket
GLU70
PHE73
ARG74
THR9
MET97
|
D Pocket
GLN114
TYR155
LYS156
LEU159
GLU160
GLY99
|
E Pocket
GLN114
GLU147
ALA152
LYS156
MET97
|
F Pocket
ALA116
ILE123
ARG143
TRP146
GLU147
LEU77
LEU80
LEU81
TYR84
GLN95
|
Colour key
Data provenance
|
1. Beta 2 microglobulin
Beta 2 microglobulin
|
10 20 30 40 50 60
IQKTPQIQVYSRHPPENGKPNILNCYVTQFHPPHIEIQMLKNGKKIPKVEMSDMSFSKDW 70 80 90 SFYILAHTEFTPTETDTYACRVKHDSMAEPKTVYWDRDM |
|
2. Class I alpha
H2-Db
|
10 20 30 40 50 60
PHSMRYFETAVSRPGLEEPRYISVGYVDNKEFVRFDSDAENPRYEPRAPWMEQEGPEYWE 70 80 90 100 110 120 RETQKAKGQEQWFRVSLRNLLGYYNQSAGGSHTLQQMSGCDLGSDWRLLRGYLQFAYEGR 130 140 150 160 170 180 DYIALNEDLKTWTAADMAAQITRRKWEQSGAAEHYKAYLEGECVEWLHRYLKNGNATLLR 190 200 210 220 230 240 TDSPKAHVTHHPRSKGEVTLRCWALGFYPADITLTWQLNGEELTQDMELVETRPAGDGTF 250 260 270 QKWASVVVPLGKEQNYTCRVYHEGLPEPLTLRWEP |
|
3. Peptide
|
LSLRNPILV
|
Data provenance
Sequences are retrieved via the Uniprot method of the RSCB REST API. Sequences are then compared to those derived from the PDB file and matched against sequences retrieved from the IPD-IMGT/HLA database for human sequences, or the IPD-MHC database for other species. Mouse sequences are matched against FASTA files from Uniprot. Sequences for the mature extracellular protein (signal petide and cytoplasmic tail removed) are compared to identical length sequences from the datasources mentioned before using either exact matching or Levenshtein distance based matching.
Downloadable data
Components
Data license
Footnotes
- Protein Data Bank Europe - Coordinate Server
- 1HHK - HLA-A*02:01 binding LLFGYPVYV at 2.5Å resolution - PDB entry for 1HHK
- Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. - PyMol CEALIGN Method - Publication
- PyMol - PyMol.org/pymol
- Levenshtein distance - Wikipedia entry
- Protein Data Bank Europe REST API - Molecules endpoint
- 3Dmol.js: molecular visualization with WebGL - 3DMol.js - Publication
- Protein Data Bank Europe REST API - Publication endpoint
- PubMed Central Europe REST API - Articles endpoint

This work is licensed under a Creative Commons Attribution 4.0 International License.